Wide range of experience
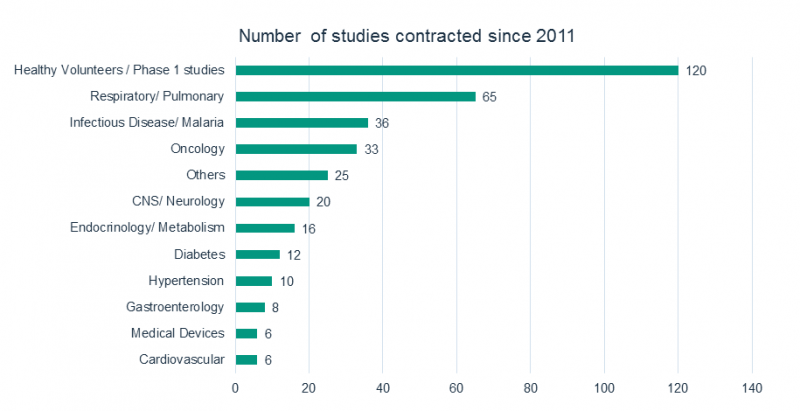
DATAMAP supports you in projects of all sizes from small phase I trials up to complete development projects within a wide spectrum of therapeutic areas/ indications.
DATAMAP has successfully performed data management and statistical services for multinational and regional clinical trials for pharmaceutical, biotechnical and medical device companies.
Profit from DATAMAP's experience since 1992, e.g. in
Clinical Trials Phase 1 - 4
Investigator Initiated Trials (IITs)
Pediatric Clinical Trials
Medical Device Trials
Non-Interventional Studies
Observational Studies, Post-Authorisation Safety Studies
and in a broad range of therapeutic areas: